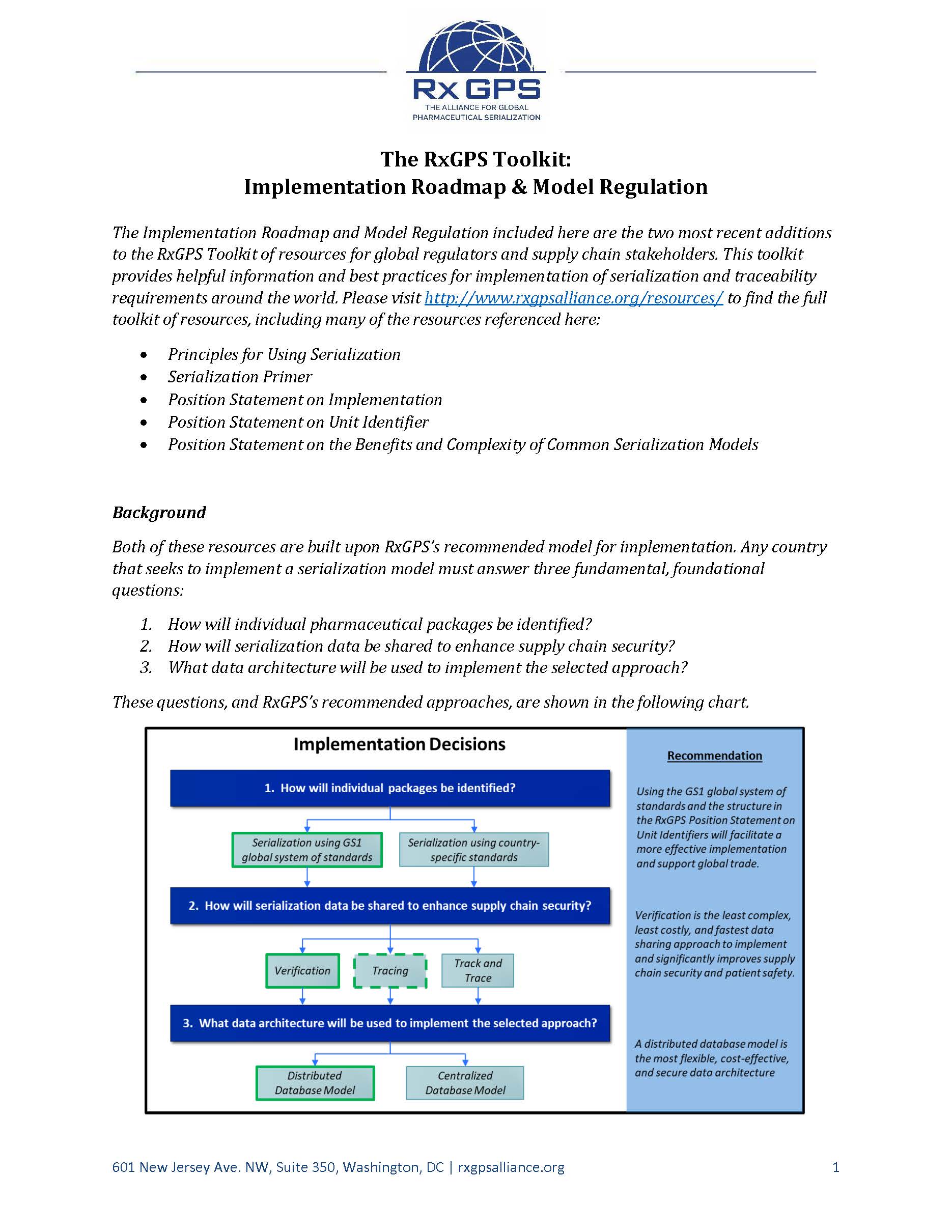
The Implementation Roadmap and Model Regulation are two resources for global regulators and supply chain stakeholders looking to develop and implement pharmaceutical serialization requirements to advance supply chain security. The Implementation Roadmap builds on the prior Position Statement on Implementation to add detail to the implementation process, particularly the regulatory process, for markets that have not yet begun that process. The Model Regulation is intended for use by global regulators in development and implementation of pharmaceutical serialization requirements, and is designed to be amended to account for individual market dynamics.